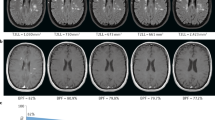
Abstract
Central nervous system (CNS) atrophy provides valuable additional evidence of an ongoing neurodegeneration independent of lesion accrual in persons with multiple sclerosis (PwMS). However, there are limitations for interpretation of CNS volume changes at individual patient-level. Patients are receiving information on the topic of atrophy through various sources, including media, patient support groups and conferences, and discussions with their providers. Whether or not the topic of CNS atrophy should be proactively discussed with PwMS during office appointments is currently controversial. This commentary/perspective article represents perspectives of PwMS, providers and researchers with recommendations for minimizing confusion and anxiety, and facilitating proactive discussion about brain atrophy, as an upcoming routine measure in evaluating disease progression and treatment response monitoring. The following recommendations were created based on application of patient’s and provider’s surveys, and various workshops held over a period of 2 years: (1) PwMS should receive basic information on understanding of brain functional anatomy, and explanation of inflammation and neurodegeneration; (2) the expertise for atrophy measurements should be characterized as evolving; (3) quality patient education materials on these topics should be provided; (4) the need for standardization of MRI exams has to be explained and communicated; (5) providers should discuss background on volumetric changes, including references to normal aging; (6) the limitations of brain volume assessments at an individual-level should be explained; (7) the timing and language used to convey this information should be individualized based on the patient’s background and disease status; (8) a discussion guide may be a very helpful resource for use by providers/staff to support these discussions; (9) understanding the role of brain atrophy and other MRI metrics may elicit greater patient satisfaction and acceptance of the value of therapies that have proven efficacy around these outcomes; (10) the areas that represent possibilities for positive self-management of MS symptoms that foster hope for improvement should be emphasized, and in particular regarding use of physical and mental exercise that build or maintain brain reserve through increased network efficiency, and (11) an additional time during clinical visits should be allotted to discuss these topics, including creation of specific educational programs.
Similar content being viewed by others
Change history
27 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00415-022-11540-x
References
Wattjes MP, Ciccarelli O, Reich DS et al (2021) 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol 20:653–670
Bermel RA, Bakshi R (2006) The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 5:158–170
Sastre-Garriga J, Pareto D, Rovira A (2017) Brain atrophy in multiple sclerosis: clinical relevance and technical aspects. Neuroimaging Clin N Am 27:289–300
Rocca MA, Battaglini M, Benedict RH et al (2017) Brain MRI atrophy quantification in MS: from methods to clinical application. Neurology 88:403–413
Azevedo CJ, Pelletier D (2016) Whole-brain atrophy: ready for implementation into clinical decision-making in multiple sclerosis? Curr Opin Neurol 29:237–242
Zivadinov R, Jakimovski D, Gandhi S et al (2016) Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 16:777–793
Sastre-Garriga J, Pareto D, Battaglini M et al (2020) MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol 16:171–182
Eshaghi A, Marinescu RV, Young AL et al (2018) Progression of regional grey matter atrophy in multiple sclerosis. Brain 141:1665–1677
Azevedo CJ, Cen SY, Khadka S, et al (2018) Thalamic atrophy in MS: an MRI marker of neurodegeneration throughout disease. Ann Neurol 83(2):223–234
Zivadinov R, Reder A, Filippi M et al (2008) Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology 71:136–144
Brand J, Kopke S, Kasper J et al (2014) Magnetic resonance imaging in multiple sclerosis–patients’ experiences, information interests and responses to an education programme. PLoS ONE 9:e113252
Munn Z, Jordan Z (2011) The patient experience of high technology medical imaging: a systematic review of the qualitative evidence. JBI Libr Syst Rev 9:631–678
Carlsson S, Carlsson E (2013) “The situation and the uncertainty about the coming result scared me but interaction with the radiographers helped me through”: a qualitative study on patients’ experiences of magnetic resonance imaging examinations. J Clin Nurs 22:3225–3234
Engels K, Schiffmann I, Weierstall R et al (2019) Emotions towards magnetic resonance imaging in people with multiple sclerosis. Acta Neurol Scand 139:497–504
Bross M, Hackett M, Bernitsas E (2020) Approved and emerging disease modifying therapies on neurodegeneration in multiple sclerosis. Int J Mol Sci 21(12):4312
Andravizou A, Dardiotis E, Artemiadis A et al (2019) Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Auto Immun Highlights 10:7
Genovese AV, Hagemeier J, Bergsland N et al (2019) Atrophied brain T2 lesion volume at MRI is associated with disability progression and conversion to secondary progressive multiple sclerosis. Radiology 293:424–433
Miller DH, Barkhof F, Frank JA, Parker GJ, Thompson AJ (2002) Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125:1676–1695
Wang C, Beadnall HN, Hatton SN, et al (2016) Automated brain volumetrics in multiple sclerosis: a step closer to clinical application. J Neurol Neurosurg Psychiatry 87(7):754–757
Beadnall HN, Wang C, Van Hecke W, Ribbens A, Billiet T, Barnett MH (2019) Comparing longitudinal brain atrophy measurement techniques in a real-world multiple sclerosis clinical practice cohort: towards clinical integration? Ther Adv Neurol Disord 12:1756286418823462
Smith SM, Zhang Y, Jenkinson M et al (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17:479–489
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE (2012) Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp 33:1987–2002
De Stefano N, Stromillo ML, Giorgio A et al (2016) Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 87:93–99
Dwyer MG, Hagemeier J, Bergsland N et al (2018) Establishing pathological cut-offs for lateral ventricular volume expansion rates. Neuroimage Clin 18:494–501
Uher T, Vaneckova M, Krasensky J et al (2019) Pathological cut-offs of global and regional brain volume loss in multiple sclerosis. Mult Scler 25:541–553
De Stefano N, Arnold DL (2015) Towards a better understanding of pseudoatrophy in the brain of multiple sclerosis patients. Mult Scler 21:675–676
Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J (2007) A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry 78:610–614
Narayanan S, Nakamura K, Fonov VS, et al (2020) Brain volume loss in individuals over time: source of variance and limits of detectability. Neuroimage 214:116737
Uher T, Bergsland N, Krasensky J et al (2021) Interpretation of Brain Volume Increase in Multiple Sclerosis. J Neuroimaging 31:401–407
Dwyer MG, Silva D, Bergsland N et al (2017) Neurological software tool for reliable atrophy measurement (NeuroSTREAM) of the lateral ventricles on clinical-quality T2-FLAIR MRI scans in multiple sclerosis. Neuroimage Clin 15:769–779
Jacobsen C, Hagemeier J, Myhr KM et al (2014) Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry 85:1109–1115
Minagar A, Barnett MH, Benedict RH et al (2013) The thalamus and multiple sclerosis: Modern views on pathologic, imaging, and clinical aspects. Neurology 80:210–219
Amiri H, de Sitter A, Bendfeldt K et al (2018) Urgent challenges in quantification and interpretation of brain grey matter atrophy in individual MS patients using MRI. Neuroimage Clin 19:466–475
Bernitsas E, Bao F, Seraji-Bozorgzad N et al (2015) Spinal cord atrophy in multiple sclerosis and relationship with disability across clinical phenotypes. Mult Scler Relat Disord 4:47–51
Daams M, Steenwijk MD, Schoonheim MM, et al (2016) Multi-parametric structural magnetic resonance imaging in relation to cognitive dysfunction in long-standing multiple sclerosis. Mult Scler 22(5):608–619
Petracca M, Pontillo G, Moccia M, et al (2021) Neuroimaging correlates of cognitive dysfunction in adults with multiple sclerosis. Brain Sci 11(3):346
Schiffmann I, Freund M, Vettorazzi E et al (2020) Assessing the effect of an evidence-based patient online educational tool for people with multiple sclerosis called UMIMS-understanding magnetic resonance imaging in multiple sclerosis: study protocol for a double-blind, randomized controlled trial. Trials 21:1008
Efendi H, Boz C, Karabudak R (2018) Evaluating treatment decision for multiple sclerosis: real life and patient experiences. Noro Psikiyatr Ars 55:S10–S14
Rudick RA, Fisher E, Lee JC, Duda JT, Simon J (2000) Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a. Mult Scler 6:365–372
De Stefano N, Sormani MP, Stubinski B et al (2012) Efficacy and safety of subcutaneous interferon beta-1a in relapsing-remitting multiple sclerosis: further outcomes from the IMPROVE study. J Neurol Sci 312:97–101
Calabresi PA, Kieseier BC, Arnold DL et al (2014) Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol 13:657–665
Filippi M, Rovaris M, Inglese M et al (2004) Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet 364:1489–1496
De Stefano N, Comi G, Kappos L et al (2014) Efficacy of subcutaneous interferon beta-1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry 85:647–653
O’Connor P, Filippi M, Arnason B et al (2009) 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol 8:889–897
Wolinsky JS, Narayana PA, Johnson KP (2001) United States open-label glatiramer acetate extension trial for relapsing multiple sclerosis: MRI and clinical correlates. Multiple Sclerosis Study Group and the MRI Analysis Center. Mult Scler 7:33–41
Rovaris M, Comi G, Rocca MA et al (2007) Long-term follow-up of patients treated with glatiramer acetate: a multicentre, multinational extension of the European/Canadian double-blind, placebo-controlled. MRI-monitored trial Mult Scler 13:502–508
Comi G, Martinelli V, Rodegher M et al (2009) Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 374:1503–1511
Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R, Group GS (2013) Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann Neurol 73:705–713
Kappos L, Wiendl H, Selmaj K et al (2015) Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 373:1418–1428
Hauser SL, Bar-Or A, Comi G et al (2017) Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 376:221–234
Arnold DL, Gold R, Kappos L et al (2014) Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J Neurol 261:1794–1802
Miller DH, Fox RJ, Phillips JT et al (2015) Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 CONFIRM study. Neurology 84:1145–1152
Miller AE, Wolinsky JS, Kappos L et al (2014) Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 13:977–986
Zivadinov R, Dwyer MG, Carl E et al (2020) Slowing of brain atrophy with teriflunomide and delayed conversion to clinically definite MS. Ther Adv Neurol Disord 13:1756286420970754
O’Connor P, Wolinsky JS, Confavreux C et al (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365:1293–1303
Radue EW, Sprenger T, Gaetano L et al (2017) Teriflunomide slows BVL in relapsing MS: a reanalysis of the TEMSO MRI data set using SIENA. Neurol Neuroimmunol Neuroinflamm 4:e390
Kappos L, Radue EW, O’Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Calabresi PA, Radue EW, Goodin D et al (2014) Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13:545–556
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Lublin F, Miller DH, Freedman MS et al (2016) Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 387:1075–1084
De Stefano N, Giorgio A, Battaglini M et al (2018) Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler 24:222–226
Leist TP, Comi G, Cree BA et al (2014) Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol 13:257–267
Kappos L, Bar-Or A, Cree BAC et al (2018) Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 391:1263–1273
Comi G, Kappos L, Selmaj KW et al (2019) Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol 18:1009–1020
Cohen JA, Comi G, Selmaj KW et al (2019) Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol 18:1021–1033
Kappos L, Fox RJ, Burcklen M et al (2021) Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial. JAMA Neurol 78:558–567
Radue EW, Stuart WH, Calabresi PA et al (2010) Natalizumab plus interferon beta-1a reduces lesion formation in relapsing multiple sclerosis. J Neurol Sci 292:28–35
Cohen JA, Coles AJ, Arnold DL et al (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380:1819–1828
Coles AJ, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380:1829–1839
Montalban X, Hauser SL, Kappos L et al (2017) Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 376:209–220
Acknowledgements
We would like to acknowledge contribution of Tracie Jacquemin and Linda Safran to this study.
Funding
There is nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Penny Pennington received consulting fee from EMD Serono. Dejan Jakimovski, Katherine Sacca, Marc Stecker, Carol B. Schumacher, Niels Bergsland, Alexis Lizarraga and Patricia Picco have nothing to disclose. Bianca Weinstock-Guttman received honoraria as a speaker and/or as a consultant for Biogen Idec, Sanofi &Genzyme, Genentech, Novartis, BMS, Bayer, Horizon and Janssen. Dr Weinstock-Guttman received research funds from Biogen Idec, Genentech and Novartis. Ralph HB. Benedict has received consultation or speaking fees from Bristol Myer Squibb, Biogen, Merck, EMD Serono, Roche, Verasci, Immune Therapeutics, Novartis, and Sanofi-Genzyme Channa Kolb has received speaker honoraria and consultant fees from EMD Serono, Teva Pharmaceuticals, Acorda, Novartis, Genzyme, Alexion, Genentech, Mallinckrodt and Biogen-Idec. Ralph HB Benedict received research support from Biogen, Bristol Meyers Squibb, Genzyme, Genentech, Novartis, National Institutes of Health, National Multiple Sclerosis Society, Verasci. He conslts for Immunic Therapeutics, Latin American Committee for Treatment and Research in Multiple Sclerosis, Merck, Novartis, Roche, Sanofi. He is on speaker bureau for Biogen, Bristol Meyers Squibb, EMD Serono. He received royalties from Psychological Assessment Resources. Svetlana Eckert Michael G. Dwyer has received personal compensation from Keystone Heart for consultant fees. He received financial support for research activities from Bristol Myers Squibb, Mapi Pharma, Keystone Heart, Protembis and V-WAVE Medical. Evanthia Bernitsas received research grants from Roche/Genentech, BMS, Sanofi/Genzyme, EMD Serono, Alexion, Novartis, TG Therapeutics, PCORI, Prime Education. She received consulting fee/honoraria: Biogen, Janssen and Janssen, Horizon Pharmaceuticals, Roche/Genentech, Greenwich Biosciences. Rana Zabad is a speaker for BMS and Genentech and a principal investigator on studies sponsored by Adamas Pharmaceuticals, Genentech, GW Pharma, Merck, Sanofi, and the TREAT-MS PCORI trial. She has served as a consultant or advisor to Bayer, Biogen Idec, Bristol Myers Squibb, Genentech, GW PHarma, Janssen Pharmaceuticals, Sanofi, and TG Therapeutics and was a member of the adjudication committee on the safety and efficacy of biotin in progressive MS by MedDay Pharmaceuticals. Gabriel Pardo has served on advisory boards and/or speakers’ bureau for Biogen Idec, Celgene/Bristol Myers Squibb, EMD Serono, Greenwich Biosciences, Janssen Pharmaceuticals, Novartis Pharmaceuticals, Roche/Genentech, Sanofi-Genzyme, TG Therapeutics and Viela Bio/Horizon Therapeutics; and is a member of the Scientific Advisory Board of Progentec Diagnostics Inc. Donald Negroski received fees as a speaker/consultant for: Alexion, Biogen, Bristol Myers Squibb, EMD -Serono, Gennetech, Janson, Novartis, Sanofi-Genzyme, TG Therapeutics. Martin Belkin has received honoraria for speaking and consulting from Biogen, Genentech, TG therapeutics, Alexion, Sanofi, EMD Serono and Bristol Myers Squibb. He has received research funds from Biogen, Sanofi, Genentech, EMD Serono and Abbvie. David Hojnacki received speaking and consulting fees from BMS, Biogen and Novartis. Robert Zivadinov has received personal compensation from Bristol Myers Squibb, EMD Serono, Sanofi, Keystone Heart, Protembis and Novartis for speaking and consultant fees. He received financial support for research activities from Sanofi, Novartis, Bristol Myers Squibb, Octave, Mapi Pharma, Keystone Heart, Protembis and V-WAVE Medical.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pennington, P., Weinstock-Guttman, B., Kolb, C. et al. Communicating the relevance of neurodegeneration and brain atrophy to multiple sclerosis patients: patient, provider and researcher perspectives. J Neurol 270, 1095–1119 (2023). https://doi.org/10.1007/s00415-022-11405-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11405-3