Updated: June 7, 2021
Summary
In an oxygen-pinching technique developed and refined on a model-driven, oxygen-consuming Human Patient Simulator, the patient or a caregiver manually interrupts oxygen flow from a cylinder during exhalation by pinching the oxygen tubing with pliers. Simulated oxygen saturation (SpO2) improved from 85% at room air to 91% while decreasing oxygen usage by up to 64% compared to continuous oxygen flow.
Description
We simulated a hypothetical acute oxygen shortage in a low resource environment encountering disruption in international air transportation where it would help if a finite source of oxygen such as an oxygen cylinder can be made to last as long as possible.
We used the HPS, an oxygen-consuming patient simulator, to determine if we could manually simulate the function of an oxygen conserver. “An oxygen conserving device provides a ‘bolus’ or burst of oxygen during the inspiratory phase of a typical breathing cycle.” (Apria manual). Cotes described in The Lancet (1956) an oxygen-conserving system requiring the patient to manually activate a control valve connected to the oxygen tubing leading to the face mask.
In the context of a hypothetical acute oxygen shortage where equipment is scarce and air transportation is disrupted, experiments were performed to determine if manually interrupting the oxygen flow without equipment during exhalation conserves oxygen while providing adequate oxygenation.
Prior experiments determined that:
- Simply pinching or kinking the tubing carrying oxygen to the face mask using only one’s hand and fingers did not interrupt O2 flow (even when doubling the tubing back onto itself) because the anti-kink O2 tubing has internal ridges that are designed to prevent interruption of O2 flow if the line is kinked.
- Crimping the tubing with a pair of pliers was less fatiguing and provided better flow interruption than kinking with one’s hand and fingers, but it was not a total interruption of oxygen flow.
- Crimping the tubing close to the facemask resulted in accumulation and compression of oxygen during exhalation in the tubing upstream of the deliberate obstruction.
- Measuring O2 cylinder usage by measuring and comparing the time it took for cylinder pressure, as indicated by the pressure gauge attached to the O2 cylinder, to drop by 200 psi showed no difference in O2 usage between continuous and interrupted O2 flow, likely because the circular scale on the pressure gauge may not be perfectly linear across the entire pressure range.
- The push fit connection of the facemask tubing inlet to the flow meter popped out at O2 flow rates greater than 10 LPM when the tubing was crimped.
Experimental protocol
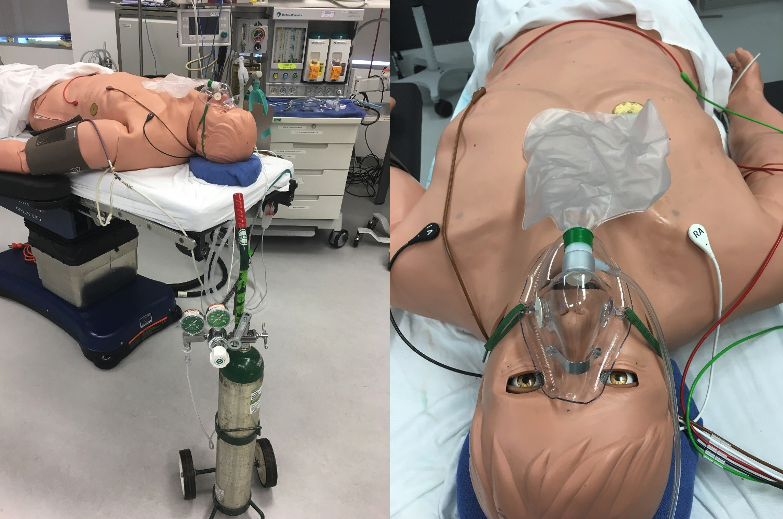
The Human Patient Simulator was allowed to breathe room air until the PaO2 stabilized at around 55 mm Hg.
Then, an O2 E-cylinder was weighed on a digital scale. For a duration of 14 minutes, the HPS was provided via a non-rebreather facemask an O2 flow from the E-cylinder of 10 LPM continuously. The E-cylinder was closed and weighed.
Next, the Human Patient Simulator was allowed to breathe room air until the PaO2 stabilized at around 55 mm Hg. Then, an O2 E-cylinder was weighed on a digital scale. For a duration of 14 minutes, the HPS was provided, via a non-rebreather facemask, an O2 flow from the E-cylinder of 10 LPM that was manually interrupted during exhalation. The flow interruption during exhalation was by manually squeezing a pair of pliers crimping the tubing immediately downstream of the flowmeter. The E-cylinder was then closed and weighed.
A leak test (tubing placed under water with O2 flowing) was performed on the area where the tubing was crimped after all experiments.
Results
| O2 Usage [g] | O2 Usage Reduction vs. Continuous O2 Flow (%) | Average Simulated PAO2 [mm Hg] | Average Simulated PaO2 [mm Hg] | Average Simulated SpO2 [%] | |
| Breathing room air | – | 100 | 131 | 53 | 85 |
| Continuous oxygen flow | 179 | – | 607 | 72 | 93 |
| Interrupted O2 flow during exhalation – SL | 64 | 64 | 294 | 61 | 88 |
| Interrupted O2 flow during exhalation – YA | 66 | 63 | 325 | 60 | 89 |
| Interrupted O2 flow during exhalation – TD | 87 | 51 | 424 | 65 | 90 |
No leak was found when testing the tubing where it was crimped by the pliers.
Limitations
The experiments are performed on a simulator – the rise in simulated alveolar partial pressure of oxygen while interrupting oxygen flow during exhalation in a simulator may not be reproducible in humans; manual interruption of O2 flow may not maintain adequate oxygen saturation (SpO2) in humans.
Only three runs (n=3) were performed for the interrupted supplemental oxygen mode.
The manual interruption was performed for 14 minutes – user fatigue may set in for longer periods.
The efficacy of the interruption technique is operator-dependent (compare simulated PA O2 with SL vs TD) and subject to human error.
The theoretical reduction of oxygen usage by 67% at an inspiratory:expiratory time ratio of 1:2 was not achieved.
An O2 flow of 10 LPM (used in the experiment) may not be adequate to maintain oxygenation in COVID patients. At O2 flowrates higher than 10 LPM, consider using a zip tie to secure the O2 tubing inlet to outlet of the flowmeter and using soapy water at the connection to verify there are no leaks at high flow rates.
The O2 tubing may develop a leak when repeatedly crimped for periods of time longer than 28 minutes.
Other factors such as the facemask fit on the face of the HPS may have changed (in spite of our attempts to only vary the supplemental O2 mode between runs).
Conclusion
In a simulator, oxygen usage was reduced by as much as 64% in the best run, while increasing alveolar partial pressure (PAO2) from a room air baseline of ~130 mm Hg to an average PAO2 of 348 mm Hg (compared to an average PAO2 of 607 mm Hg with continuous O2 flow) when oxygen flow of 10 LPM was interrupted manually during exhalation.
It is important to note that the oxygen utilization from the cylinder was reduced by intermittent occlusion but there was a penalty in terms of a reduced simulated PAO2. The simulated PAO2 still increased substantially compared with the baseline at room air and increased with time suggesting that the operator technique improved. For patients with profound respiratory failure, maximizing the PAO2 becomes important and the reduced PAO2 with oxygen flow interruption may still be clinically important.
Materials
- Human Patient Simulator (Version B, CAE Healthcare, Sarasota, FL)
- Oxygen (O2) source: O2 E cylinder pressure regulator and flowmeter; Western Enterprises M1 Series Flow Gauge Oxygen Regulator, MPN: M1-870-15FG
- Non-rebreather face mask with O2 inlet, bag and tubing (Teleflex, 1059)
- Pliers (Harborfreight, 4-3/4 in. flat nose pliers)
- Digital scale (Model EK-12Ki, 12,000 g × 1 g, A&D Engineering, San Jose, CA)
- Timer (Apple, iPhone 8)
- Soapy water (to detect potential leaks)
Human Patient Simulator (HPS) settings
- Set to simulate a spontaneously breathing tachypneic adult patient
- Respiratory rate 30 breaths per minute
- Inspiratory to expiratory time ratio 1:2
- Tidal Volume 500 mL
- Shunt Fraction: 0.48 (to achieve a simulated arterial partial pressure, PaO2, of approximately 55 mm Hg)
Supplemental oxygen mode
- O2 flowmeter set to deliver 10 liters per minute (LPM) 100% O2 from E-cylinder and allowed to flow continuously
- O2 flowmeter set to deliver 10 liters per minute (LPM) 100% O2 from E-cylinder but O2 flow is manually interrupted during each exhalation by crimping the kink-resistant O2 tubing with pliers immediately downstream of the O2 flowmeter
References
Oxygen Conserving Devices. Patient/Caregiver Instructions. Apria Healthcare. Accessed May 8, 2021. https://www.apria.com/wp-content/uploads/2014/10/RES-2007-Manual_O2-Consv-Dev_07-14_v19_online.pdf
Cotes JE, Gilson JX. Effect of oxygen on exercise ability in chronic respiratory insufficiency use of portable apparatus. Lancet 1956;1:872–876.