Development of an In Vitro Blink Model for Ophthalmic Drug Delivery
Abstract
:1. Introduction
2. Methods
2.1. Contact Lenses
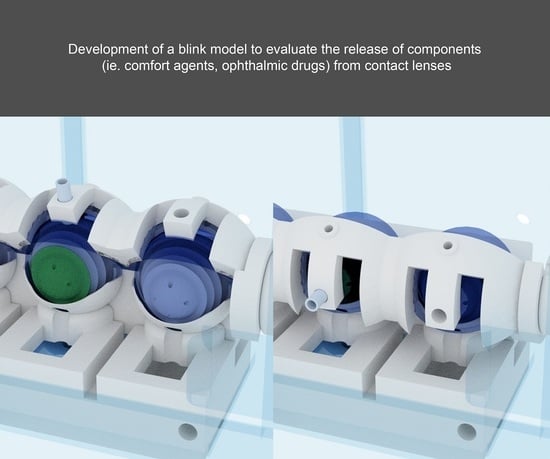
2.2. Blink Model
2.3. Flow and Blink Speed
2.4. Uptake and Release Study
2.5. Extraction of Dye from Lenses and Eyelid
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartung, T.; Daston, G. Are in Vitro Tests Suitable for Regulatory Use? Toxicol. Sci. 2009, 111, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.; Senchyna, M.; Glasier, M.-A.; Schickler, J.; Forbes, I.; Louie, D.; May, C. Lysozyme and Lipid Deposition on Silicone Hydrogel Contact Lens Materials. Eye Contact Lens 2003, 29, S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, H.; Heynen, M.; Trieu, D.; Hagedorn, S.J.; Jones, L. The Impact of Tear Film Components on in Vitro Lipid Uptake. Optom. Vis. Sci. 2012, 89, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B. A 3-µW Cmos Glucose Sensor for Wireless Contact-Lens Tear Glucose Monitoring. IEEE J. Solid State Circuits 2012, 47, 335–344. [Google Scholar] [CrossRef]
- Ng, A.; Heynen, M.; Luensmann, D.; Subbaraman, L.N.; Jones, L. Optimization of a Fluorescence-Based Lysozyme Activity Assay for Contact Lens Studies. Curr. Eye Res. 2013, 38, 252–259. [Google Scholar] [CrossRef]
- Walther, H.; Lorentz, H.; Heynen, M.; Kay, L.; Jones, L.W. Factors That Influence in Vitro Cholesterol Deposition on Contact Lenses. Optom. Vis. Sci. 2013, 90, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, H.; Subbaraman, L.; Jones, L.W. In Vitro Cholesterol Deposition on Daily Disposable Contact Lens Materials. Optom. Vis. Sci. 2016, 93, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, H.; Heynen, M.; Kay, L.M.M.; Dominici, C.Y.; Khan, W.; Ng, W.W.S.; Jones, L. Contact Lens Physical Properties and Lipid Deposition in a Novel Characterized Artificial Tear Solution. Mol. Vis. 2011, 17, 3392–3405. [Google Scholar] [PubMed]
- Lorentz, H.; Heynen, M.; Khan, W.; Trieu, D.; Jones, L. The Impact of Intermittent Air Exposure on Lipid Deposition. Optom. Vis. Sci. 2012, 89, 1574–1581. [Google Scholar] [CrossRef]
- Bhatt, P.; Narvekar, P.; Lalani, R.; Chougule, M.B.; Pathak, Y.; Sutariya, V. An in Vitro Assessment of Thermo-Reversible Gel Formulation Containing Sunitinib Nanoparticles for Neovascular Age-Related Macular Degeneration. AAPS PharmSciTech 2019, 20, 281. [Google Scholar] [CrossRef]
- Walther, H.; Phan, C.M.; Subbaraman, L.N.; Jones, L. Differential Deposition of Fluorescently Tagged Cholesterol on Commercial Contact Lenses Using a Novel in Vitro Eye Model. Transl. Vis. Sci. Technol. 2018, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Phan, C.-M.; Walther, H.; Subbaraman, L.N.; Jones, L. Depth Profile Assessment of the Early Phase Deposition of Lysozyme on Soft Contact Lens Materials Using a Novel in Vitro Eye Model. Eye Contact Lens 2018, 44, S11–S18. [Google Scholar] [CrossRef]
- Phan, C.M.; Walther, H.; Smith, R.W.; Riederer, D.; Lau, C.; Lorenz, K.O.; Subbaraman, L.N.; Jones, L. Determination of the Release of Peg and Hpmc from Nelfilcon a Daily Disposable Contact Lenses Using a Novel in Vitro Eye Model. J. Biomater. Sci. Polym. Ed. 2018, 29, 2124–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, C.M.; Walther, H.; Gao, H.; Rossy, J.; Subbaraman, L.N.; Jones, L. Development of an in Vitro Ocular Platform to Test Contact Lenses. J. Vis. Exp. 2016, 110, e53907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, C.M.; Bajgrowicz-Cieslak, M.; Subbaraman, L.N.; Jones, L. Release of Moxifloxacin from Contact Lenses Using an in Vitro Eye Model: Impact of Artificial Tear Fluid Composition and Mechanical Rubbing. Transl. Vis. Sci. Technol. 2016, 5, 3. [Google Scholar] [CrossRef]
- Phan, C.M.; Bajgrowicz, M.; Gao, H.; Subbaraman, L.N.; Jones, L.W. Release of Fluconazole from Contact Lenses Using a Novel in Vitro Eye Model. Optom. Vis. Sci. 2016, 93, 387–394. [Google Scholar] [CrossRef]
- Bajgrowicz, M.; Phan, C.M.; Subbaraman, L.N.; Jones, L. Release of Ciprofloxacin and Moxifloxacin from Daily Disposable Contact Lenses from an in Vitro Eye Model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2234–2242. [Google Scholar] [CrossRef]
- Tieppo, A.; Pate, K.M.; Byrne, M.E. In Vitro Controlled Release of an Anti-Inflammatory from Daily Disposable Therapeutic Contact Lenses under Physiological Ocular Tear Flow. Eur. J. Pharm. Biopharm. 2012, 81, 170–177. [Google Scholar] [CrossRef]
- Ali, M.; Horikawa, S.; Venkatesh, S.; Saha, J.; Hong, J.W.; Byrne, M.E. Zero-Order Therapeutic Release from Imprinted Hydrogel Contact Lenses within in Vitro Physiological Ocular Tear Flow. J. Control. Release 2007, 124, 154–162. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; McBride, M.K.; Pate, K.M.; Tieppo, A.; Byrne, M.E. Extended Release of High Molecular Weight Hydroxypropyl Methylcellulose from Molecularly Imprinted, Extended Wear Silicone Hydrogel Contact Lenses. Biomaterials 2011, 32, 5698–5705. [Google Scholar] [CrossRef]
- Lu, Q.; Yin, H.; Grant, M.P.; Elisseeff, J.H. An in Vitro Model for the Ocular Surface and Tear Film System. Sci. Rep. 2017, 7, 6163. [Google Scholar] [CrossRef]
- Shafaie, S.; Hutter, V.; Cook, M.T.; Brown, M.B.; Chau, D.Y.S. In Vitro Cell Models for Ophthalmic Drug Development Applications. Biores. Open Access 2016, 5, 94–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.; Byun, W.Y.; Alisafaei, F.; Georgescu, A.; Yi, Y.S.; Massaro-Giordano, M.; Shenoy, V.B.; Lee, V.; Bunya, V.Y.; Huh, D. Multiscale Reverse Engineering of the Human Ocular Surface. Nat. Med. 2019, 25, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Walther, H.; Qiao, H.; Shinde, R.; Jones, L. Development of an Eye Model with a Physiological Blink Mechanism. Transl. Vis. Sci. Technol. 2019, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Phan, C.M.; Qiao, H.; Yee, A.; Jones, L. Deposition of Fluorescently Tagged Lysozyme on Contact Lenses in a Physiological Blink Model. Eye Contact Lens 2021, 47, 127–133. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, X.; Qi, P. Therapeutic Contact Lenses for Ophthalmic Drug Delivery: Major Challenges. J. Biomater. Sci. Polym. Ed. 2020, 31, 549–560. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Anguiano-Igea, S.; Varela-García, A.; Vivero-Lopez, M.; Concheiro, A. Bioinspired Hydrogels for Drug-Eluting Contact Lenses. Acta Biomater. 2019, 84, 49–62. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A Comprehensive Review on Contact Lens for Ophthalmic Drug Delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Hui, A. Contact Lenses for Ophthalmic Drug Delivery. Clin. Exp. Optom. 2017, 100, 494–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Mathews, J.; Leake, K.; Bell, J. Factors Affecting Evaporation Rates of Tear Film Components Measured in Vitro. Eye Contact Lens 2009, 35, 32–37. [Google Scholar] [CrossRef]
- Calonge, M.; Pinto-Fraga, J.; González-García, M.J.; de Salamanca, A.E.; de la Rosa, A.L.; Fernández, I.; López-Miguel, A. Effects of the External Environment on Dry Eye Disease. Int. Ophthalmol. Clin. 2017, 57, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, R.E.; Polse, K.A. Changes in Tear Flow Accompanying Aging. Am. J. Optom. Physiol. Opt. 1978, 55, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Subbaraman, L.N.; Jones, L. In Vitro Drug Release of Natamycin from Beta-Cyclodextrin and 2-Hydroxypropyl-Beta-Cyclodextrin-Functionalized Contact Lens Materials. J. Biomater. Sci. Polym. Ed. 2014, 25, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Subbaraman, L.; Liu, S.; Gu, F.; Jones, L. In Vitro Uptake and Release of Natamycin Dex-B-Pla Nanoparticles from Model Contact Lens Materials. J. Biomater. Sci. Polym. Ed. 2014, 25, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Subbaraman, L.N.; Jones, L. In Vitro Uptake and Release of Natamycin from Conventional and Silicone Hydrogel Contact Lens Materials. Eye Contact Lens 2013, 39, 162–168. [Google Scholar] [CrossRef] [PubMed]


 ) etafilcon A in a vial, (
) etafilcon A in a vial, (  ) senofilcon A in a vial, (
) senofilcon A in a vial, (  ) etafilcon A on the eye model, and (
) etafilcon A on the eye model, and (  ) senofilcon A on the eye model (n = 3).
) senofilcon A on the eye model (n = 3).
 ) etafilcon A in a vial, (
) etafilcon A in a vial, (  ) senofilcon A in a vial, (
) senofilcon A in a vial, (  ) etafilcon A on the eye model, and (
) etafilcon A on the eye model, and (  ) senofilcon A on the eye model (n = 3).
) senofilcon A on the eye model (n = 3).
 ) etafilcon A in a vial, (
) etafilcon A in a vial, (  ) senofilcon A in a vial, (
) senofilcon A in a vial, (  ) etafilcon A on the eye model, and (
) etafilcon A on the eye model, and (  ) senofilcon A on the eye model (n = 3).
) senofilcon A on the eye model (n = 3).
 ) etafilcon A in a vial, (
) etafilcon A in a vial, (  ) senofilcon A in a vial, (
) senofilcon A in a vial, (  ) etafilcon A on the eye model, and (
) etafilcon A on the eye model, and (  ) senofilcon A on the eye model (n = 3).
) senofilcon A on the eye model (n = 3).


| Categories | 1-Day Acuvue Moist | 1-Day Acuvue OASYS |
|---|---|---|
| USAN | etafilcon A | senofilcon A |
| Manufacturer | Johnson & Johnson | Johnson & Johnson |
| Center thickness, mm | 0.07 | 0.085 |
| Water content, % | 58 | 38 |
| Oxygen permeability, ×10−11 | 28 | 125 |
| FDA group | IV | V (C) |
| Principal monomers | HEMA, PVP, MA | mPDMS+DMA+HEMA+siloxane macromer +TEGDMA+PVP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, C.-M.; Shukla, M.; Walther, H.; Heynen, M.; Suh, D.; Jones, L. Development of an In Vitro Blink Model for Ophthalmic Drug Delivery. Pharmaceutics 2021, 13, 300. https://doi.org/10.3390/pharmaceutics13030300
Phan C-M, Shukla M, Walther H, Heynen M, Suh D, Jones L. Development of an In Vitro Blink Model for Ophthalmic Drug Delivery. Pharmaceutics. 2021; 13(3):300. https://doi.org/10.3390/pharmaceutics13030300
Chicago/Turabian StylePhan, Chau-Minh, Manish Shukla, Hendrik Walther, Miriam Heynen, David Suh, and Lyndon Jones. 2021. "Development of an In Vitro Blink Model for Ophthalmic Drug Delivery" Pharmaceutics 13, no. 3: 300. https://doi.org/10.3390/pharmaceutics13030300