New EASL CPGs provide guidance on haemochromatosis
1 June 2022
EASL is proud to announce the release of EASL Clinical Practice Guidelines on Haemochromatosis, just published in the Journal of Hepatology. This marks World Haemochromatosis Week taking place every year in the first week of June.
The Panel was chaired by Heinz Zoller. Panel members included Annick Vanclooster, Bill Griffiths, Edouard Bardou-Jacquet, Elena Corradini, Graça Porto, John Ryan, Benedikt Schaefer, and EASL Governing Board representative, Markus Cornberg.
Why are these CPGs valuable to the hepatology community?
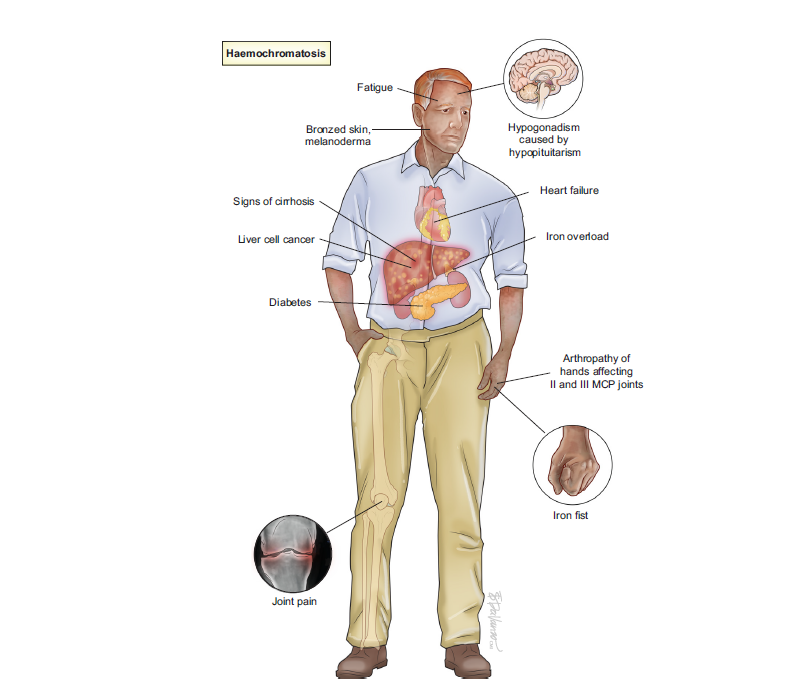
Haemochromatosis is considered the commonest genetic disease in adults and affects ca. 1 in 1,000 Europeans. If untreated, haemochromatosis can cause fatigue, progress to cirrhosis, hepatocellular carcinoma, and joint disease. Early diagnosis and treatment can prevent these complications. In the light of recent progress in understanding the disease pathogenesis, and improvements in diagnosis, disease definitions had to be adjusted to appropriately to diagnose the disease, select patients for treatment and optimise treatment, counselling and follow up, which is all delivered in this new guideline.
EASL has addressed the topic of haemochromatosis in previous Clinical Practice Guidelines of 2010, also published in the Journal of Hepatology. This 2022 publication is a profound revision of the previous CPGs.
Who is the target audience of these CPGs?
These CPGs are intended for healthcare professionals actively involved in the care and management of patients with haemochromatosis. The target audience is thus not limited to hepatologists, but is also pertinent to haematologists, rheumatologists, transfusion medicine specialists, and general practitioners. The guidelines are also intended for patients themselves, living with haemochromatosis.
These CPGs provide evidence-based guidance on care for patients with haemochromatosis, while making practical recommendations in important areas of patient care where less evidence exists. The latter was derived from scientific discussions among experts in the field which was further improved by incorporating suggestions of a larger group of experts in a structured Delphi process. All recommendations are graded by their level of evidence and strength of recommendation. The CPGs are based on important clinical questions and the results are summarised in comprehensive algorithms for everyday use in clinical practice,
said Prof. Heinz Zoller, Chair of the panel.
A snapshot of these CPGs
These CPGs cover practical clinical questions on how to select patients for genetic testing, how to interpret these test results, and which differential diagnoses to consider. In patients with an established diagnosis, recommendations on disease staging, treatment, and follow-up are provided. As an example, recommendations on how to manage and best care for patients with ambiguous results from genetic testing (e.g. compound heterozygosity for p.C282Y/H63D in HFE) are provided.
What are the core contents of these CPGs?
- What should be included in an iron panel to test patients for suspected haemochromatosis?
- Who should be tested for the p.C282Y variant in HFE?
- In whom should non-invasive tests for quantification of iron overload be performed?
- When is a liver biopsy required in patients with haemochromatosis?
- How and when should fibrosis stage be assessed?
- Which extrahepatic manifestations should be investigated?
- Which patients should undergo screening for HCC and how often?
- When should rare haemochromatosis gene variants be tested?
- Haemochromatosis management in pregnancy
- What is the first-line treatment in patients with haemochromatosis?
- When should second-line treatment be considered?
- What are the treatment targets?
- What are the dietary recommendations in haemochromatosis?
What is the methodology behind these CPGs?
These CPGs were developed using the Delphi method, a framework based on the results of multiple rounds of questionnaires sent to a panel of experts. After the questions were phrased by the writing panel and approved by the expert panel, the writing panel held weekly online meetings. Following that, CPGs were sent to the Delphi panel, which included international experts in hepatology, gastroenterology, haematology, transfusion medicine, internal medicine, radiology, and patient representatives.